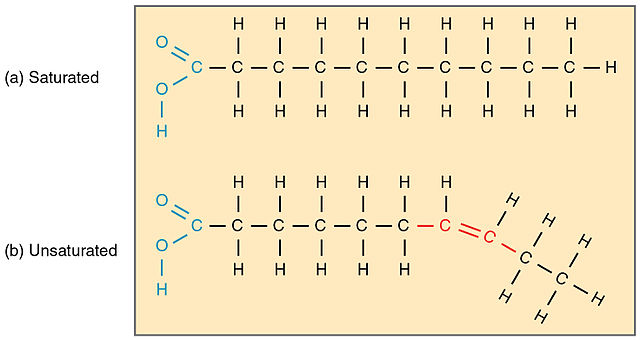
Saturated and unsaturated fatty acids
Fatty acids are classified into two groups:
- The saturated fatty acids.
- The unsaturated fatty acids.
Saturated fatty acids
In saturated fatty acids, all of their covalent bonds between carbon atoms are single bonds, so their hydrocarbon chains are linear. They are solid at room temperature.
 By BruceBlaus. When using this image in external sources it can be cited as:Blausen.com staff. "Blausen gallery 2014". Wikiversity Journal of Medicine. DOI:10.15347/wjm/2014.010. ISSN 20018762. (Own work) [CC BY 3.0], via Wikimedia Commons
By BruceBlaus. When using this image in external sources it can be cited as:Blausen.com staff. "Blausen gallery 2014". Wikiversity Journal of Medicine. DOI:10.15347/wjm/2014.010. ISSN 20018762. (Own work) [CC BY 3.0], via Wikimedia Commons They are found in animal fats, and in coconut, palm, and peanut oils.
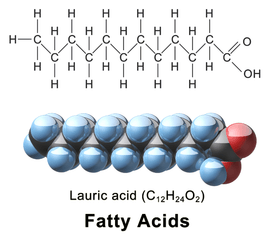
Examples of saturated fatty acids are myristic, palmitic, and stearic.
|
Saturated fatty acids |
Melting point (ºC) |
|
Myristic CH 3 - (CH 2 ) 12 - COOH |
53.9 |
|
Palmitic CH 3 - (CH 2 ) 14 - COOH |
63.1 |
|
Stearic CH 3 - (CH 2 ) 16 - COOH |
69.6 |
|
Lignoceric CH 3 - (CH 2 ) 22 - COOH |
86.0 |
The chains of the saturated fatty acids can be packed by Van der Waals bonds between the atoms of the neighboring chains. The longer the chain (more carbons), the greater the possibility of formation of these weak interactions. Therefore, at room temperature, saturated fatty acids are usually in the solid state.
Animated image: Saturated fatty acids.