Spatial isomerism or stereoisomerism
The spatial isomerism or stereoisomerism is the existence of molecules with the same molecular formula but different spatial structure, so they have different properties.
Stereoisomerism occurs whenever there is an asymmetric carbon atom.
A carbon is asymmetric when it is attached to four different radicals.
All monosaccharides, except dihydroxyacetone, have asymmetric (chiral) carbons, attached to four different chemical radicals.
The isomers are compounds having the same chemical formula but different structure.
Stereoisomers that have the same chemical properties but different optical activity are called optical isomers. Optical isomers can be:
- Enantiomers or enantiomorphs. One stereoisomer is the mirror image of the other, not superimposable.
- Diastereoisomers. Those that are not mirror images of the other.
The number of optical isomers (stereoisomers) that a biomolecule has is equal to 2n, where "n" is the number of asymmetric carbons it has.
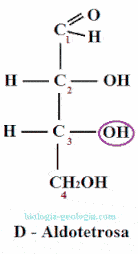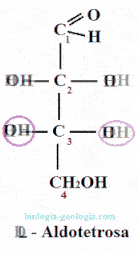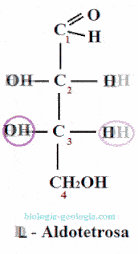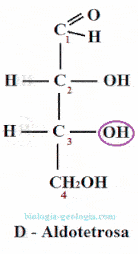
By convention, it is considered that belong to the D monosaccharides whose penultimate carbon (asymmetric carbon the farthest away from the functional group) has the group -OH to the right, and if L has left.
Most of the carbohydrates found in nature are of the D form.
For example, the second carbon atom in glyceraldehyde is asymmetric. The isomer with structure L is the non-superimposable mirror image (image in a mirror) of the isomer with structure D. Both are called enantiomers or enantiomorphs.
Two optical isomers are enantiomers or enantiomorphic when they are mirror images of each other. The position of the OHs on all asymmetric carbons varies.
Placing the aldehyde group at the top and looking at that carbon, two spatial isomers or stereoisomers can be distinguished, which only differ in the spatial position of a radical -OH:
- the D -gliceraldehído when the -OH is at the d ight .
- the L-glyceraldehyde, when the -OH is at the left.
This rule applies to the rest of the monosaccharides.
When two monosaccharides differ only in the position of the hydroxyl group on a carbon, they are said to be epimers. For example, as we will see, D-erythrose is an epimer at carbon 2 of D-treose.
The epimers are stereoisomers wherein only varies the position of the group -OH of one of the asymmetric carbons.
The presence of asymmetric carbons gives these molecules the property of optical activity (or rotational power). When a beam of polarized light falls on them when they are in aqueous solution, a deviation of the plane of vibration of that beam occurs. They are distinguished:
- Right-handed molecules, if they deviate to the right (like clockwise), and are represented by the sign (+). For example, since D-glyceraldehyde is dextrorotatory, it is called D - (+) glyceraldehyde.
- Left-handed molecules, if they deviate to the left, and are symbolized by the sign (-).
The right-handed (+) form and the left-handed (-) form are called optical isomers. The angle of deviation of the light they produce is the same, although in the opposite direction. The enantiomorphic structures are thus optical isomers.
Whether a monosaccharide is dextrorotatory or left-handed is completely independent of whether it belongs to the D or L form.
The dihydroxyacetone is the only monosaccharide has no asymmetric carbon atom and, therefore, no optical activity.
The enantiomorphic structures differ from each other in the position of all the -OH radicals of the asymmetric carbons. They are the same substance, with the same properties, except in optical activity.