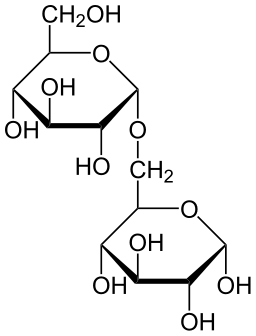
Disaccharides
Disaccharides are formed by the union of two monosaccharides through an O-glucosidic bond, which can be done in two ways:
- By monocarbonyl bond between the anomeric carbon of the first monosaccharide and any non-anomeric carbon of the second. As an anomeric carbon remains with the free hemiacetal, it continues to have the reducing capacity. For example, the maltose, the cellobiose and lactose. The name ending of the first monosaccharide is -osil and that of the second monosaccharide is -ose.

By Javier Velasco (Own work) [CC BY-SA 4.0], via Wikimedia Commons
- By dicarbonyl bonding, when the two anomeric carbons of the two monosaccharides are involved, thereby losing the reducing capacity, such as sucrose. The name ending of the first monosaccharide is -osil and that of the second monosaccharide is -oside.
Disaccharides have the same properties as monosaccharides: they are soluble in water, crystallizable, colorless and sweet in taste. Its reducing capacity depends on whether it has a free anomeric group.
The main disaccharides of biological interest are:
- Maltose.
- Cellobiose.
- Lactose.
- Saccharose o sucrose.
- Isomalt.
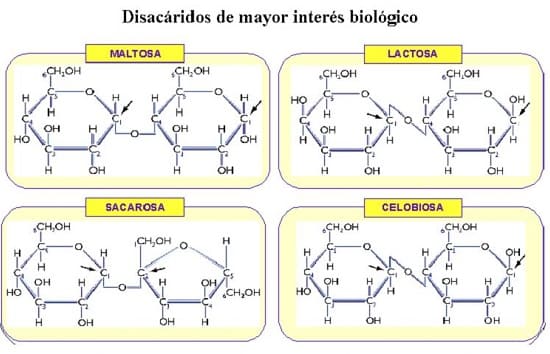
http://biogeo.iespedrojimenezmontoya.es/BIOLOGIAJM/BIOQUIMICA/TEMA3GLUCIDOS.htm, CC BY-SA 4.0, via Wikimedia Commons