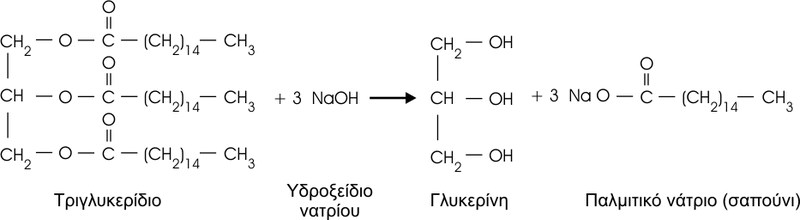
The saponification reaction is a typical reaction of fatty acids, in which they react with a strong base (NaOH or KOH) and give rise to a fatty acid salt, called soap.
Soap molecules have amphipathic behavior, with a lipophilic or hydrophobic zone, which avoids contact with water, and a hydrophilic or polar zone, which tends to bind to water.
For example, a soap such as sodium palmitate (CH3-(CH2)14-COONa), has a lipophilic zone, the hydrocarbon chain, which establishes Van der Waals bonds with lipophilic molecules. The hydrophilic zone (-COONa) is ionized, leaving the carboxyl group with a negative electric charge (-COO-), establishing electric-type attractions with water molecules and other polar groups . For this reason, they do not form true solutions, but rather they constitute colloidal dispersions, forming micelles., which can be monolayers, or bilayers if they include water inside.