Phospholipids
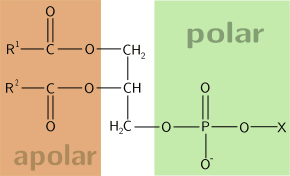
The phospholipids are a class of compounds by a lipid molecule alcohol (glycerol or sphingosine), which bind two fatty acids and a phosphate group. Phosphate is linked by a phosphodiester bond to another group of atoms, which generally contain nitrogen, such as choline, serine or ethanolamine and often have an electrical charge.
Phospholipids have an amphipathic behavior, since they present:
- a polar, hydrophilic zone, formed by the phosphate group attached to an alcohol,
- an apolar, lipophilic zone in which two fatty acids appear.
By No machine-readable author provided. Lennert B assumed (based on copyright claims). [Public domain], via Wikimedia Commons
This amphipathic behavior allows them to form lipid bilayers, the main component of the plasma membrane, where the apolar tails of both layers face each other, while the polar heads are oriented towards the external and internal environment, both aqueous.

By Rupertsciamenna. Versión en español de Alejandro Porto. (File:Fosfolipide.svg) [CC BY-SA 3.0], via Wikimedia Commons
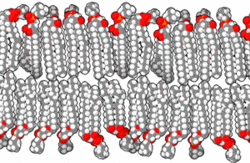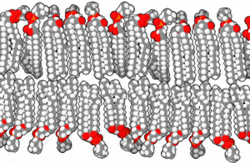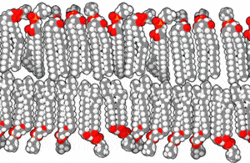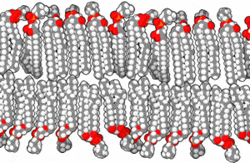
By Bensaccount [Public domain], via Wikimedia Commons
The phospholipids are divided into:
- Phosphoglycerides ( alcohol is glycerol, a short-chain alcohol).
- Sphingolipids (alcohol is sphingosine, a long-chain alcohol).

