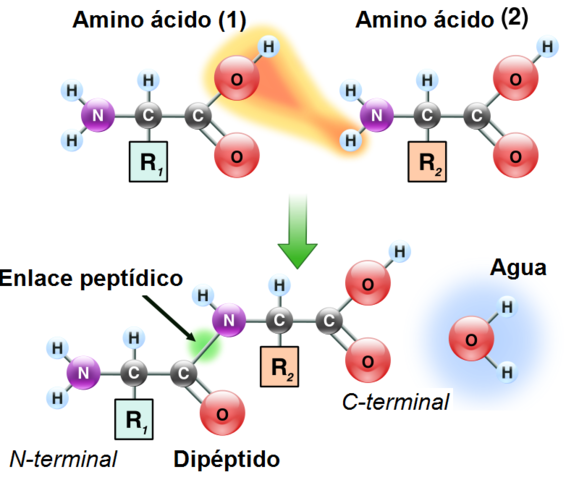
The two ends of a peptide are not equivalent, since it has an N-terminal and a C-terminal end. By convention, the amino terminus is considered the beginning of the peptide chain.
The peptide bond is stabilized because the carbon, oxygen and nitrogen atoms share electrons, which causes two resonant forms to appear and that the bond between carbon and nitrogen has a partial double bond character.
Due to this partial double bond character of the peptide bond, the arrangement in space of the four atoms of the peptide unit and the two alpha carbon atoms is such that they lie in the same plane (peptide plane) with fixed distances and angles; this planar arrangement is rigid. There is only freedom of rotation around the alpha carbons.
The radicals of the amino acids do not participate in the peptide bonds, being arranged up and down, alternately, in the polypeptide,
The main characteristics of the peptide bond are:
- It is an amide-type bond between the carboxyl group of one amino acid and the amino of another with the release of a water molecule, but it is shorter than other bonds.
- The peptide bond has a partial double bond character, which is why it is rigid and does not show twist, which causes the atoms involved (C and N, as well as O and H) to lie in the same plane.
- The peptide bond presents polarity with the appearance of charge densities in O and N. This property is responsible for the appearance of H bonds between peptide bonds, responsible for the secondary structure of proteins.