Secondary structure of proteins
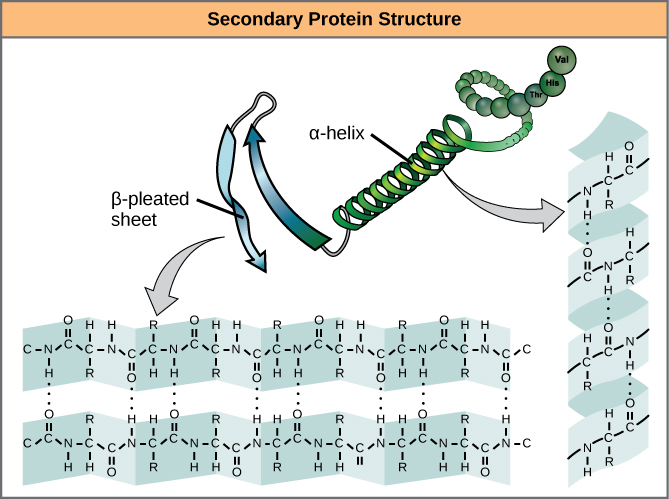
The secondary structure of a protein is the arrangement in space of the amino acids sequence or primary structure. It is due to the rotational capacity of the bonds that form the α-carbon, and to the radicals of the amino acids, which make the polypeptide acquire a stable spatial arrangement, the secondary structure.
The secondary structure of the polypeptide chain depends on the amino acids that make it up.
It is the highest structural level that fibrous proteins reach.
Three types of secondary structure are known:
- The α helix.
- The folded sheet or β sheet.
- The collagen helix.