N-glycosidic and O-glucosidic bonds
There are two types of bonds between a monosaccharide and other molecules:
- The N-glucosidic bond, which is formed between an -OH and an amino compound (will give rise to amino sugars).
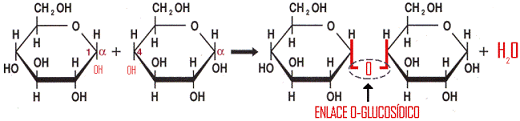
- The O-glucosidic bond, which is made between two -OH of two monosaccharides, previously cyclized, eliminating a water molecule by condensation, being at least one of the OH groups that react from the anomeric carbon (C1).
The O-glucosidic bond is α-glucosidic if the first monosaccharide is α, and β-glucosidic if the first monosaccharide is β. For example, between C1 of an α-D-glucopyranose and C4 of another D-glucopyranose (α or β) an α-type bond is established (1→4).